This kit is used for the in vitro qualitative detection of the ORF1ab gene and N gene of the new coronavirus (SARS-CoV-2) in human nasopharyngeal swabs and oropharyngeal swabs specimens of suspected cases of SARS-CoV-2 pneumonia and other people who need to be diagnosed or differentially diagnosed with SARS-CoV-2 infection.
This kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro molecular diagnostic procedures.
This kit is for in vitro diagnostic use only.
Stable
--lyophilized reagents, transportation ≤37℃, stable for 3 months.
Cost-effective
--the PCR materials are premixed. No extra equipment required.
Fast
--only takes 28 minutes with QPCR instrument to get result.
Accurate
--Specificity: ≥98% Sensitivity: ≥98% LoD: ≥100 copies/mL CV: ≤ 2.3%
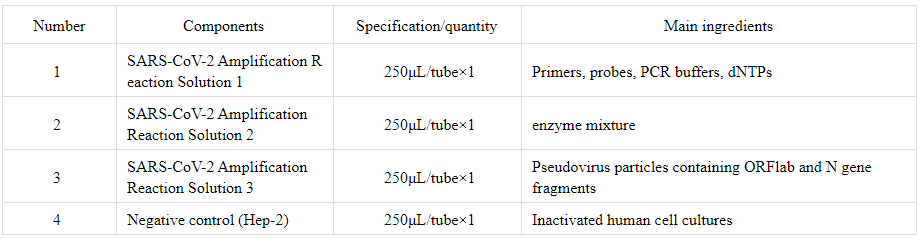
【Main components】