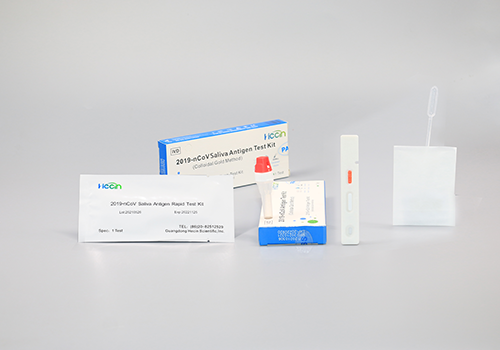
This kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of in vitro diagnostic procedures.
This kit is only used for the in vitro qualitative detection of 2019-nCoV antigen from human saliva specimens.
This kit is suitable for the auxiliary diagnosis of COVID-19, the results are for clinical reference only and cannot be used as the sole basis for diagnosis and exclusion decision. The clinical diagnosis and treatment of patients should be considered in combination with their symptoms/signs, medical history, other laboratory tests and treatment responses.
Positive test result needs to be further confirmed, negative result does not preclude 2019-nCoV infection.
The kit is immunochromatographic and uses double-antibody sandwich method to detect 2019-nCoV N protein antigen.
During detection, the treated specimens are loaded into the sample wells of the test card. When the concentration of 2019-nCoV antigen in specimen is higher than the minimum detection limit, the viral antigen will form complexes with labeled antibodies first. Under chromatography, the complexes move forward along the nitrocellulose membrane till captured by pre-coated monoclonal antibody of 2019-nCoV in detection zone on nitrocellulose film (T) to form a pink/purple reaction line on the detection zone, at this point the result is positive; conversely, if there is no viral antigen or the concentration of antigen in specimen is below the minimum detection limit, no pink/purple reaction line appears in the detection zone, at this point the result is negative.
Regardless of whether the sample contains viral antigens or not, a pink/purple reaction line will appear in the quality control zone (C), the pink/purple reaction line that appears in the quality control zone (C) is the criterion for determining if the chromatography process is normal.