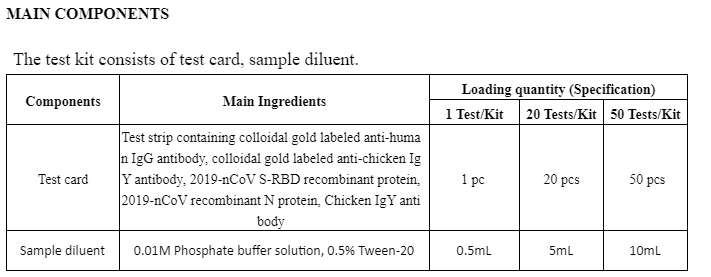
This kit is only used for the in vitro qualitative detection of 2019-nCoV S-RBD IgG antibody and N protein IgG antibody from human serum, plasma or whole blood.
This kit is intended for the detection of neutralizing antibody against COVID-19, which can better monitor herd immunity and protective immunity, and evaluate the efficacy of vaccines during clinical trials as well as after mass vaccination. The results are for clinical reference only.
This kit is intended for the distinction between the protectively immunized populations after vaccination of 2019-nCoV inactivated vaccine and subunit vaccine / mRNA vaccine. The results are for clinical reference only.
This kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques in the field of in vitro diagnostic procedures.
The kit uses immunochromatography technology and capture method to detect 2019-nCoV S-RBD IgG antibody and N protein IgG antibody.
During detection, the specimens are loaded into the sample wells of the test card. When the concentration of 2019-nCoV S-RBD IgG antibody or N protein IgG antibody in specimen is higher than the minimum detection limit, the antibody in specimen will form complexes with colloidal gold labeled anti-human IgG antibody first. Under chromatography, the complexes move forward along the nitrocellulose membrane till captured by pre-coated recombinant protein in detection zone (T) on nitrocellulose strip to form a pink/purple reaction line on the detection zone (T), at this point the result is positive; conversely, if there is no 2019-nCoV S-RBD IgG antibody or N protein IgG antibody, or the antibody concentration in specimen is below the minimum detection limit, no pink/purple reaction line appears in the detection zone (T), at this point the result is negative.
Regardless of whether specimen contains 2019-nCoV antibody or not, a pink/purple reaction line will appear in the quality control zone (C), the pink/purple reaction line that appears in the quality control zone (C) is the criterion for determining if the chromatography process is normal.
Note:
1. Test cards are sealed together with desiccant in aluminum foil pouch.
2. Do not mix use different batches of test cards and sample diluent.