Intended Use
Product features
Basic parameters
Intended Use
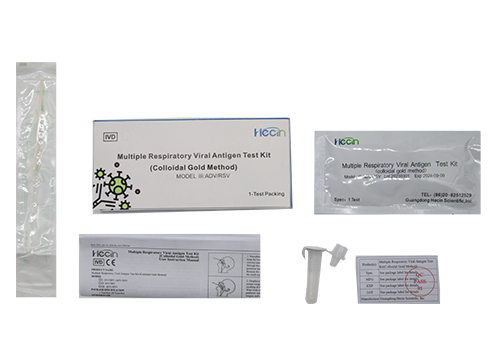
The kit is used for the in vitro qualitative detection of respiratory syncytial virus (RSV) and adenovirus (ADV) antigens in human nasopharyngeal swabs or oropharyngeal swabs specimens.
Both respiratory syncytial virus(RSV)and respiratory adenovirus(ADV) can cause respiratory infections with similar clinical symptoms, mainly fever, cough, nasal congestion, throat discomfort, fatigue, headache, muscle aches and other symptoms. Some patients are accompanied by shortness of breath, bronchitis or pneumonia.
Product features
Excellent Performance
--High accuracy and specificity
Extremly Simple Operation
--Point-of-Care diagnosis, no instrument needed
Fast Detection
--Result out within 15 minutes
Basic parameters
| Items | Parameters |
| Sample Type | Nasopharyngeal swab; oropharyngeal swabs |
| Package Specification | 1 Test/Kit; 20 Tests/Kit |
| Method | Colloidal gold method |
| Performance | ADV Accuracy: 96.30% (95%CI: 93.96%-97.91%) |
| RSV Accuracy: 99.01% (95%CI: 97.49%-99.73%) | |
| Storage/Shelf life | 2℃-30℃ for 18 months |
| Obtained certificates | CE |