Intended Use
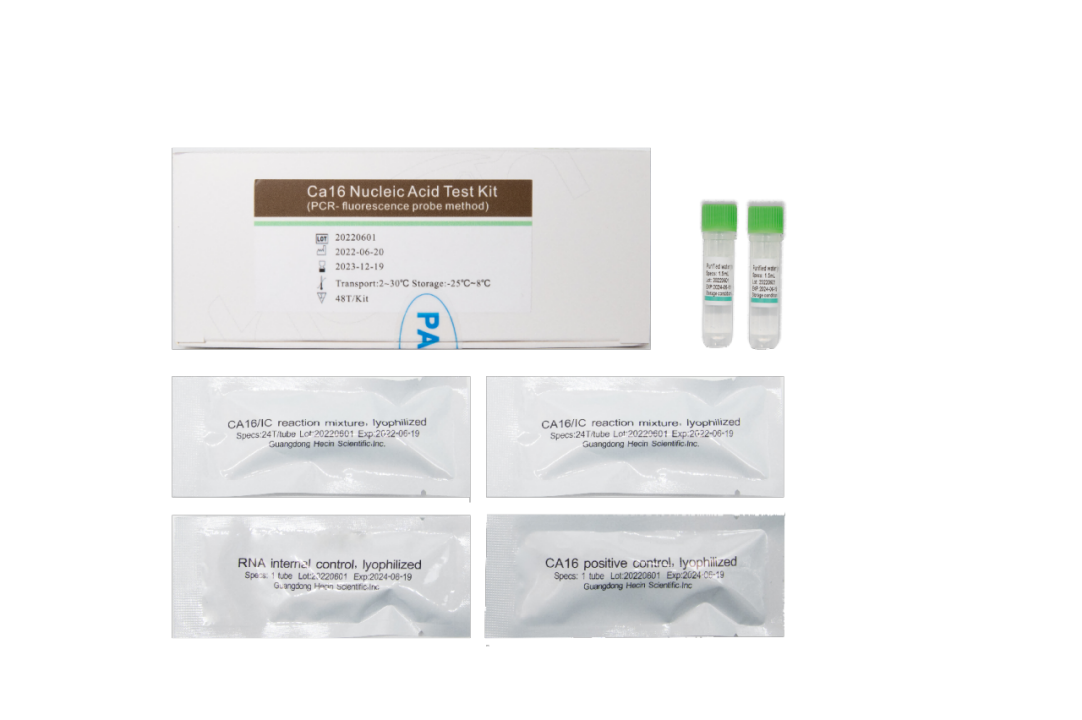
Product features
Basic parameters
Intended Use
This kit is intended for in vitro qualitative detection of Human Coxsackie virus A16 (CA16) nucleic acid in human oropharyngeal swabs specimens.
This kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro molecular diagnostic procedures.
This kit is for in vitro diagnostic use only.
Product features
Fast
----Real-time fluorescense PCR technology, from sample to result out takes 30mins only
Accurate
---- Internal control included, monitoring the whole extraction to amplification process
Easy transportation:
-----Lyophilized reagtent, can be shipped at 2~30℃ for 3 months
Basic parameters
| Items | Parameters |
| Sample type | Oropharyngeal swab |
| Specification | 48T/Kit |
| PCR instruments | HC 1600; ABI 7500 |
| LoD | 0.03 TCID50/0.1mL |
| Storage/Shelf life | -25℃to 8℃ away from light; 18 months |
| Obtained certificates | CE; NMPA |