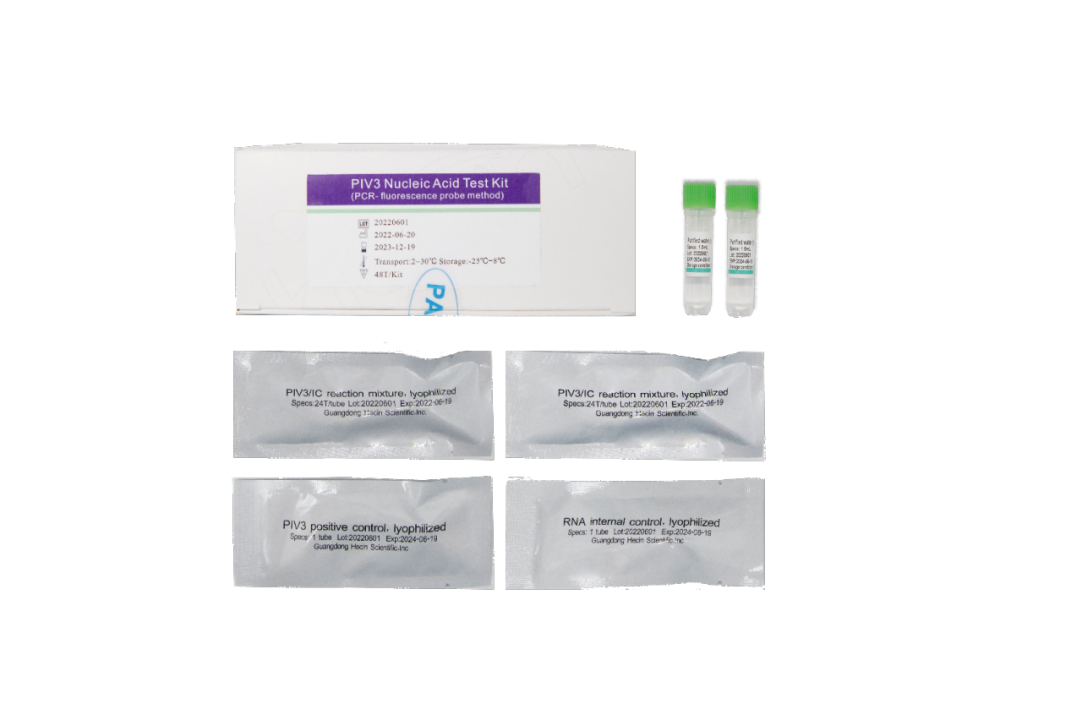
Intended Use
Product features
Basic parameters
Intended Use
This kit is intended for in vitro qualitative detection of Parainfluenza virus type 3 (PIV3) nucleic acid in human oropharyngeal swabs specimens.
This kit is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro molecular diagnostic procedures.
This kit is for in vitro diagnostic use only.
Product features
Fast
----Real-time RT-PCR technology, from sample to result out takes 30mins only
Accurate
---- Internal control included, monitoring the whole extraction to amplification process
Easy transportation:
-----Lyophilized reagtent, can be shipped at 2~30℃ for 3 months
Basic parameters
| Items | Parameters |
| Sample type | Oropharyngeal swab |
| Specification | 48T/Kit |
| PCR instruments | HC 1600; ABI 7500 |
| LoD | 0.02TCID50/0.1mL |
| Storage/Shelf life | -25℃to 8℃ away from light; 18 months |
| Obtained certificates | CE; NMPA |