Polymerase chain reaction (PCR) is a powerful technique that has transformed the field of molecular biology. Over the years, several variations of PCR have been developed to enhance its capabilities. These variations include reverse transcription PCR (RT-PCR), quantitative PCR (qPCR), and reverse transcription quantitative PCR (RT-qPCR). In this article, we will discuss the differences between these techniques and provide examples of their applications.
PCR
PCR is a technique that amplifies a specific DNA sequence by using short, synthetic oligonucleotides called primers to initiate DNA synthesis. PCR works by repeatedly heating and cooling a reaction mixture containing the DNA template, primers, and a heat-stable DNA polymerase enzyme. Each cycle of PCR doubles the amount of the targeted DNA sequence. PCR is widely used in genetic research, diagnostics, and forensics.
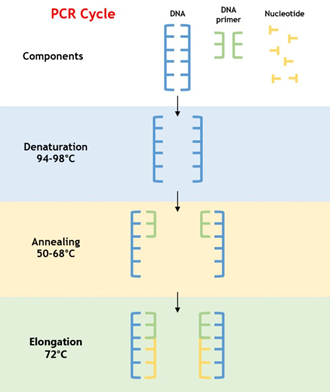
For example, PCR can be used to diagnose infectious diseases such as COVID-19. By using specific primers that target the SARS-CoV-2 virus, PCR can detect the presence of the virus in patient samples such as nasal swabs or saliva. PCR can also be used to identify genetic variations that may contribute to disease susceptibility or drug resistance.
RT-PCR
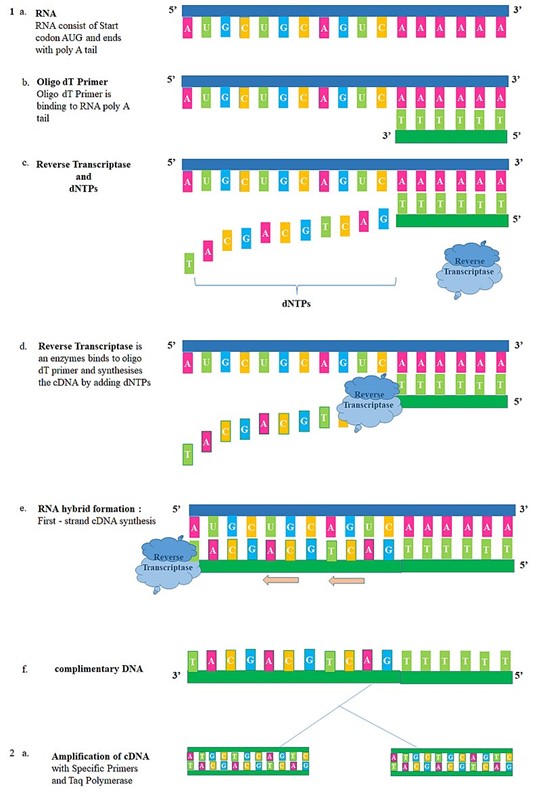
Reverse transcription PCR (RT-PCR) is a variation of PCR that is used to amplify RNA molecules. RNA is first converted to complementary DNA (cDNA) by reverse transcriptase enzyme, and then PCR is performed using cDNA as a template. RT-PCR is widely used to quantify gene expression levels, identify RNA viruses, and study RNA splicing.
For example, RT-PCR can be used to study the expression of genes that are involved in cancer progression. By extracting RNA from cancer cells and using specific primers to amplify the cDNA, researchers can measure the levels of gene expression in these cells. RT-PCR can also be used to detect RNA viruses such as influenza and HIV.
qPCR
Quantitative PCR (qPCR), also known as real-time PCR, is a variation of PCR that allows for the quantification of DNA or RNA in a sample. qPCR works by using fluorescent dyes or probes that bind to the amplified DNA sequence during PCR. As more DNA is amplified, the fluorescence signal increases, and the amount of DNA can be measured in real-time. qPCR is useful for quantifying gene expression levels, detecting pathogens, and identifying genetic variations.
For example, qPCR can be used to quantify the expression of genes that are involved in drug metabolism. By using fluorescent probes that target specific genes, researchers can measure the levels of gene expression in liver cells. qPCR can also be used to detect the presence of pathogens in clinical samples such as blood or urine.
RT-qPCR
Reverse transcription quantitative PCR (RT-qPCR) combines the reverse transcription step of RT-PCR with the quantitative capabilities of qPCR. RT-qPCR is a powerful technique for measuring gene expression levels and studying RNA splicing events. RT-qPCR can also be used to detect RNA viruses and quantify viral loads in clinical samples.
For example, RT-qPCR can be used to study the expression of genes that are involved in neurological disorders such as Alzheimer's disease. By extracting RNA from brain tissue and using specific probes to amplify the cDNA, researchers can measure the levels of gene expression in different regions of the brain. RT-qPCR can also be used to detect and quantify viral loads in patient samples, which is important for monitoring the progression of viral infections such as hepatitis C.
In conclusion, PCR, RT-PCR, qPCR, and RT-qPCR are all variations of the same basic technique that have been developed to enhance its sensitivity, specificity, and quantification capabilities. Each technique has its own unique applications and can be used to study a wide range of biological questions.






