Recently, the China Institute of Food and Drug Control has added a new national reference product for novel coronavirus antigen detection reagents. This reference product is restricted to be supplied to relevant in vitro diagnostic reagent manufacturers as a new variety for registration testing of new coronavirus antigen detection reagents. On April 16, Guangdong Hecin Scientific, Inc., as a cooperating unit, participated in the work related to the new coronavirus nucleic acid reference materials announced by the Central People's Procuratorate. For the new coronavirus antigen reagent national reference product announced this time, Guangdong Hecin once again served as a cooperating unit to participate in the work related to the new coronavirus antigen reference product.

The national reference products for COVID-19 antigen detection reagents include: 20 negative reference products, 8 positive reference products, and 1 minimum detection limit and repeatability reference product.
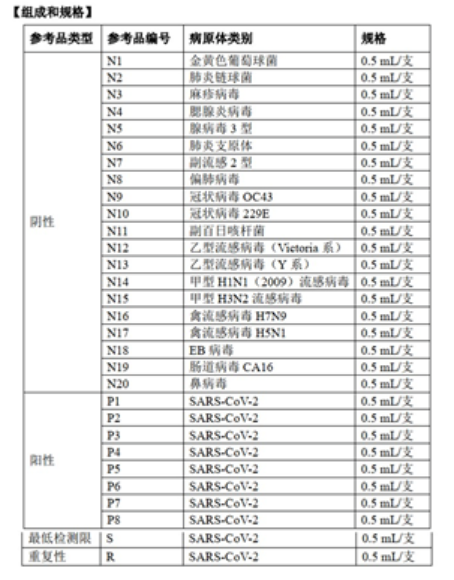
The use of reference products is as follows:
1) Positive reference product: Use pure water to dilute 1:10 before testing. All test results must be positive to be qualified.
2) Negative reference product: directly use the original multiple for testing, and all test results must be negative to be qualified.
3) Minimum detection limit reference product: Dilute the lowest detection limit reference product with pure water at 1:50, 1:100, 1:200, 1:400, 1:800 and 1:1600 and mark it as S1 ~ S6, where s1-s4 are all required to be detected, but S5 and S6 are not required;
4) Repeatable reference product: First, dilute the reference product 1:10 and 1:100 with pure water and mark it as R1 and R2. Conduct 10 repeatability tests on each of R1 and R2, and get the 10 test results of R1 and R2. They should all be positive, and the color development should be uniform without difference; or the CV of 10 test results should not be greater than 20.0%.
Only if the above 4 conditions are met at the same time can the COVID-19 antigen detection kit pass the verification of this reference product.

Congratulations again to Guangdong Hecin Scientific, Inc. as a collaborative unit for participating in the work related to the new coronavirus antigen reference product





