Hecin’s SARS-CoV-2 Antigen Test Kit has received EU CE
In the face of the new coronavirus epidemic that may further intensify in autumn and winter, the global new coronavirus epidemic is facing the pressure of a second outbreak. The situation in overseas countries fighting the epidemic is still very serious. The demand for COVID-19 testing kits remains unabated and can provide quick results. On-site antigen testing is expected to become one of the important epidemic prevention measures. Our company concentrated the superior strength of the R&D department and developed a new coronavirus (SARS-CoV-2) antigen detection kit (colloidal gold method) in a short period of time, and obtained the EU CE certification.

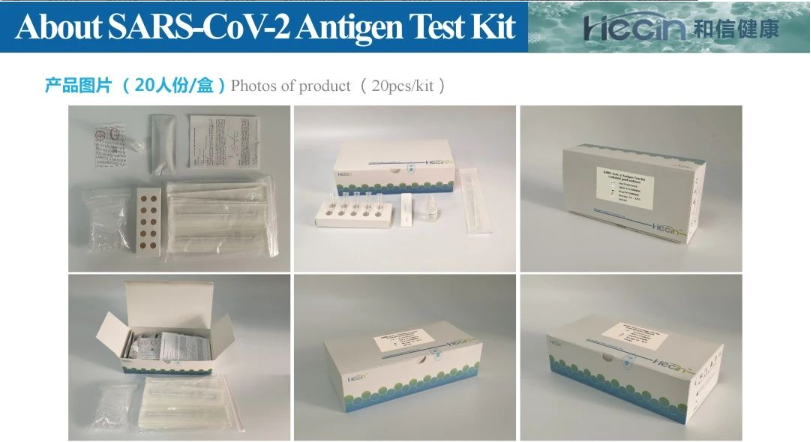

The kit is an immunochromatographic method that can directly detect whether human samples contain the new coronavirus. Diagnosis is fast, accurate, and requires less equipment and personnel. It uses a double-antibody sandwich method, using two antigen-specific antibodies to identify and combine different epitopes of a target antigen, which can greatly reduce the probability of cross-reaction, thereby effectively improving its specificity. For the in vitro qualitative detection of novel coronavirus (SARS-CoV-2) N protein antigen in human nasopharyngeal swab and oropharyngeal swab samples.
【Detection principle】
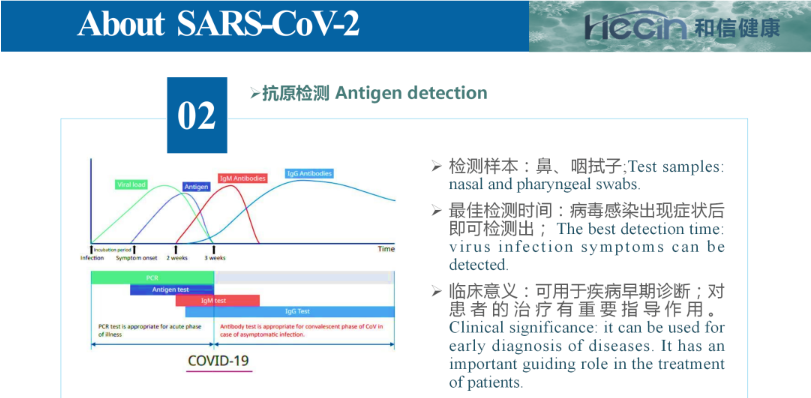
The reagent uses immunochromatography technology and uses a double-antibody sandwich method to detect novel coronavirus (SARS-CoV-2) antigens. During detection, after the processed sample to be tested is dropped into the sample hole of the test card, when the sample to be tested contains the new coronavirus (SARS-CoV-2) antigen and the antigen concentration is higher than the minimum detection limit, the viral antigen will be detected first. Forms a reaction complex with labeled antibody. Under the action of chromatography, the reaction complex moves forward along the nitrocellulose membrane and is pre-coated with the novel coronavirus (SARS-CoV) in the detection zone (T) on the nitrocellulose membrane. -2) Capture of nucleoprotein monoclonal antibodies, and finally form a red/pink reaction line in the detection area, at which time the result is positive; on the contrary, when the sample to be tested does not contain viral antigen or the antigen concentration is lower than the minimum detection limit, then the detection area There is no red/purple reaction line, so the result is negative. The quality control area (C) produces a red/purple reaction line regardless of whether the sample contains viral antigen or not. The red/purple reaction line shown in the quality control area (C) is a criterion for determining whether the chromatography process is normal. It is also used as an internal control standard for reagents.





