Intended Use
Product features
Basic parameters
Intended Use
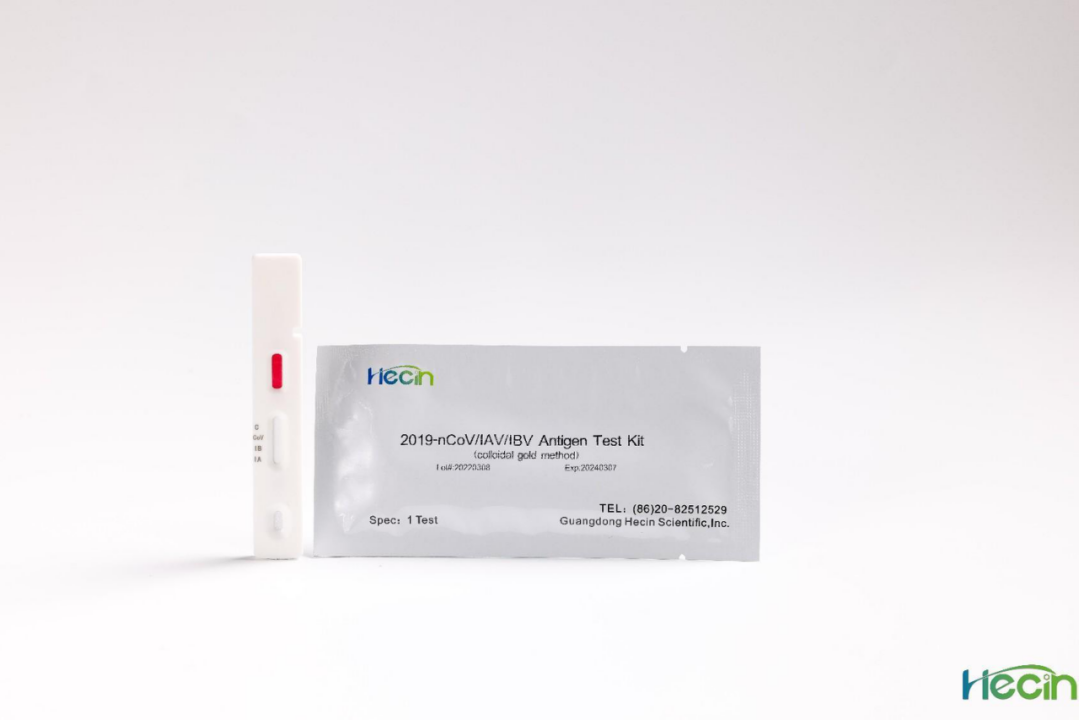
This kit is intended for the in vitro qualitative detection of antigens of 2019-nCoV, Influenza A virus (IAV) or Influenza B virus (IBV) in human nasopharyngeal swabs or oropharyngeal swabs specimens.
Product features
Fast
----result out within 15 min, Point-of-Care diagnosis, no instrument needed
User-friendly
---- extremely simple operation for detecting viral infection
Convenient in Stoarge&Shipment
-----room temperature(2℃-30℃) for 18 months
Basic parameters
| Items | Parameters |
| Sample Type | nasopharyngeal swabs ; oropharyngeal swabs specimens |
| Package Specification | 1T/Kit; 20TKit |
| Method | Collodial gold method |
| Performance | IAV Accuracy: 98.71% (95%CI: 97.36%~99.48%) |
| IBV Accuracy: 97.96% (95%CI: 96.38%~98.98%) | |
| 2019n-CoV Accuracy: 98.36% (95%CI: 96.80%~99.29%) | |
| Storage/Shelf Life | room temperature(2°C~30°C) valid for 24 months |
| Obtained Certificates | CE |