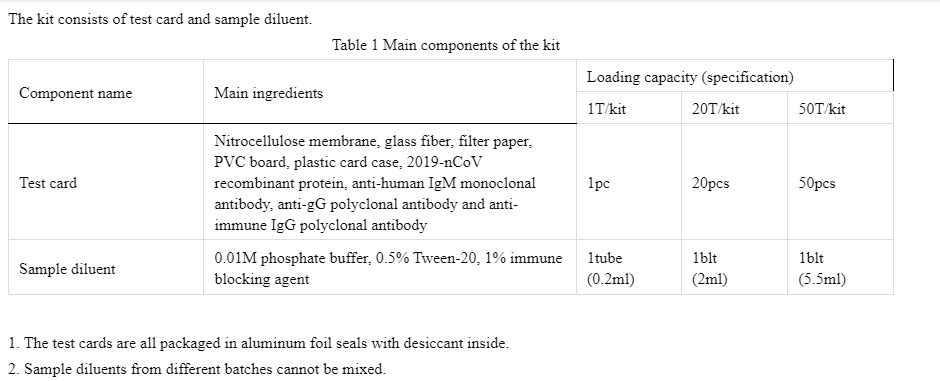
【expected usage】
This kit is used for the in vitro qualitative detection of novel coronavirus (2019-nCoV) IgM antibodies in human serum and plasma samples.
It is only used as a supplementary testing indicator for suspected cases with negative nucleic acid test results of the new coronavirus or used in conjunction with nucleic acid testing in the diagnosis of suspected cases. It cannot be used as the basis for the diagnosis and exclusion of pneumonia caused by the new coronavirus infection, and is not suitable for screening of the general population.
This kit is for use in medical institutions only. A positive test result requires further confirmation, and a negative test result cannot rule out the possibility of infection. The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the patient's condition in combination with the patient's clinical manifestations and other laboratory tests.
When carrying out laboratory testing of new coronavirus, it should comply with the requirements of the "Technical Guidelines for Laboratory Testing of Pneumonia Infected by New Coronavirus" and ensure biosafety protection.
This reagent uses immunochromatography technology and uses an indirect method to detect human novel coronavirus (2019-nCoV) IgM antibodies.
During detection, add the processed sample to be tested to the sampling hole of the test card. When the sample to be tested contains human novel coronavirus (2019-nCoV) IgM antibodies and the IgM antibody concentration is higher than the minimum detection limit, the virus IgM The antibody first forms a reaction complex with the labeled secondary antibody. Under the action of chromatography, the reaction complex moves forward along the nitrocellulose membrane and is pre-coated with the human novel coronavirus (T) in the detection area (T) on the nitrocellulose membrane. 2019-nCoV) recombinant antigen capture will eventually form a red/pink reaction line on the detection area, and the result is positive at this time; on the contrary, when the sample to be tested does not contain viral IgM antibodies or the IgM antibody concentration is lower than the minimum detection limit, If no red/purple reaction line appears in the detection area, the result is negative.
Regardless of whether the sample contains viral IgM antibodies, a red/purple reaction line will be formed in the quality control area (C). The red/purple reaction line displayed in the quality control area (C) is the standard for determining whether the chromatography process is normal. It also serves as an internal control standard for reagents.