This kit is used for the in vitro qualitative detection of the ORF1ab gene and N gene of the new coronavirus (SARS-CoV-2) in throat swab samples of suspected cases of new coronavirus pneumonia and other people who need to be diagnosed or differentially diagnosed with new coronavirus infection.
The definition of "suspected cases" and other groups shall be implemented with reference to the "Novel Coronavirus Pneumonia Diagnosis and Treatment Plan", "Novel Coronavirus Pneumonia Prevention and Control Plan" and other documents. The use of this product should comply with the relevant requirements of the "Novel Coronavirus Pneumonia Diagnosis and Treatment Case", "Novel Coronavirus Pneumonia Prevention and Control Plan" and other documents. When carrying out nucleic acid testing of new coronavirus, it should comply with the requirements of the "Technical Guidelines for Collection and Testing of New Coronavirus Samples" and ensure biosafety.
The test results of this kit are for clinical reference only and should not be used as the only standard for clinical diagnosis. It is recommended to conduct a comprehensive analysis of the patient's condition in combination with the patient's clinical mnifestations and other laboratory tests.
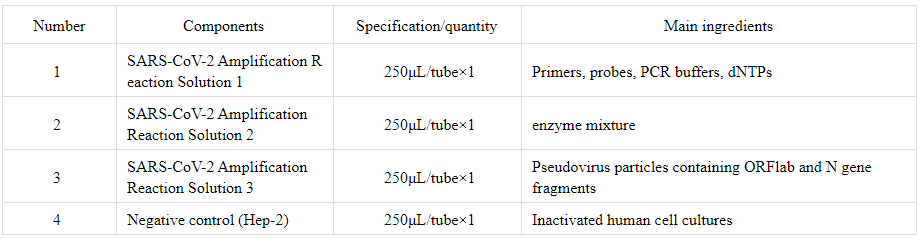
【Main components】